The Triflo valve is the result of years of research in collaboration with world leaders in aeronautics, medicine, and science. Exhaustive in-vitro and in-vivo testing performed on the valve suggest its safety and effectiveness.
57
In-vitro testing
The design of the valve has been validated by means of state-of-the-art experimental and numerical testing:
- Hydrodynamic performance
- Structural performance
- Durability and wear tests
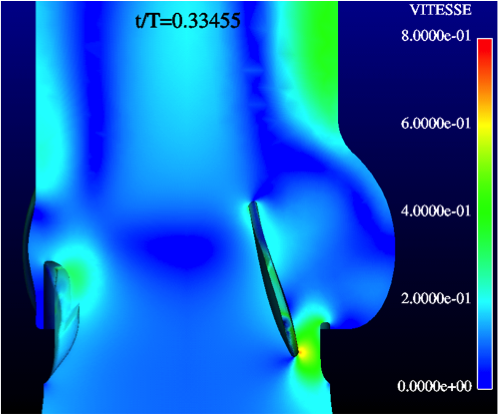
Extensive comparative in-vitro testing has been performed by independent institutes, including:
- Bern University, Artorg Center Biomedical Engineering Research (Bern, Switzerland)
- Texas A&M Engineering Experiment Station (Texas, USA)
- École Polytechnique Fédérale de Lausanne (EPFL, Switzerland)
- Swiss Federal Institute of Technology, Zürich (ETH, Switzerland)
- Vivitro Systems, Inc. (Victoria, Canada)
- Helmholtz-Institute (Aachem, Germany)
- California Institute of Technology, CALTECH (Pasadena, California, USA.)
- Simulog (Toulouse, France)
58
In-vivo testing
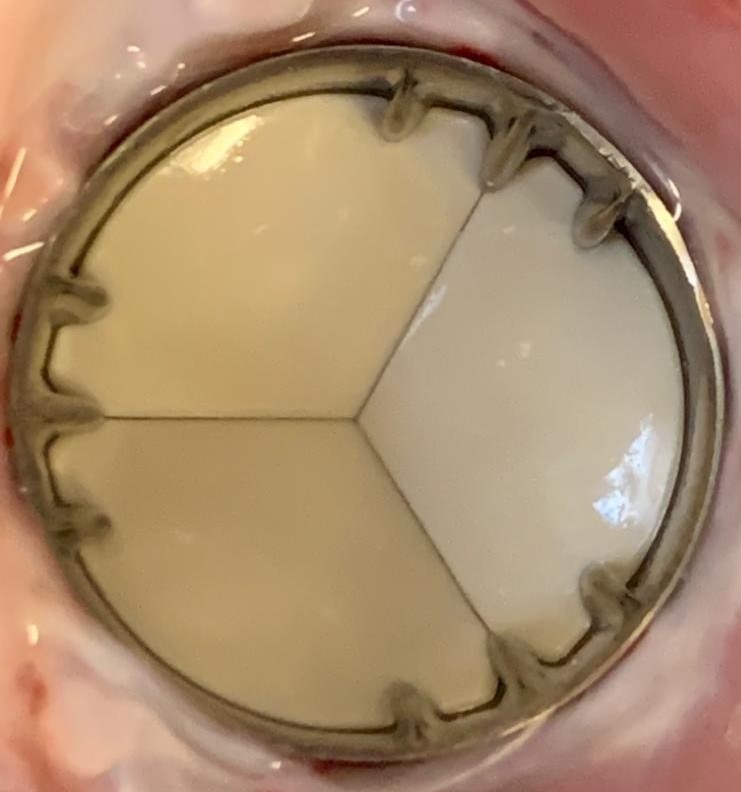
Outstanding in-vivo results after long-term implantation in the aortic, mitral, and pulmonary positions without any anticoagulant
